AI & ML in Clinical Trials: Use Cases & Challenges
AI and Machine Learning are transforming clinical trial design by improving protocol feasibility, accelerating patient recruitment, enhancing safety monitoring, increasing diversity, and reducing reliance on large control arms. This article explores the most impactful use cases, real-world examples, benefits, and key challenges of adopting AI in a regulatory-ready, scientifically sound way.

Clinical trial design is reaching a breaking point. Protocols are longer and more complex, patient populations are narrower, and the volume of clinical and real-world data continues to grow. At the same time, enrolment delays, protocol amendments, and high dropout rates are driving timelines and costs to unsustainable levels.
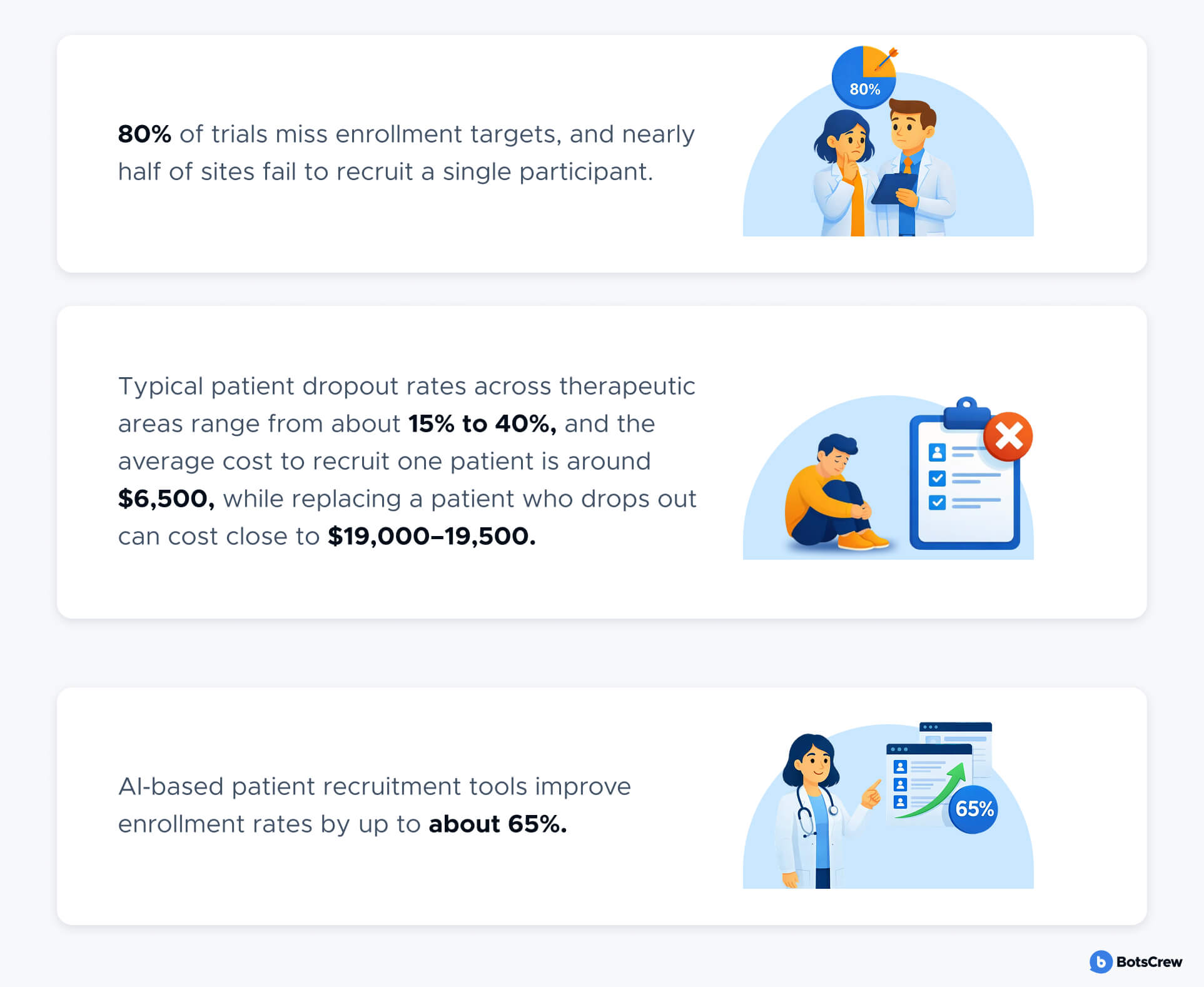
Today, more than 80% of trials fail to meet enrollment targets, nearly half of sites recruit no patients, and replacing a single dropout can cost close to $20,000. Recruitment and retention alone account for roughly 30% of total drug development timelines, with delays costing large programs millions of dollars per day.
Artificial Intelligence (AI) and Machine Learning (ML) are no longer experimental tools in this environment. They are becoming core decision-support technologies for sponsors seeking to design trials that are:
- More feasible and predictable
- More patient-centric
- More statistically robust
- More aligned with regulatory expectations
- Less exposed to operational and safety risk
Leading organizations are already using AI to simulate protocol feasibility, forecast recruitment, optimize eligibility criteria, identify high-performing sites, improve diversity, monitor safety signals earlier, and reduce reliance on large control arms.
Book an AI Readiness Assessment for Your Clinical Programs
Understand where AI can deliver the fastest ROI in your protocol design, recruitment, and safety workflows.
For clinical operations and digital leaders, the question is no longer if AI can support trial design, but where it can safely deliver measurable impact today—and how to adopt it in a regulatory-ready, scientifically sound way.
AI vs. ML in Clinical Trials — Quick Definitions
Artificial Intelligence (AI) refers to systems that perform tasks traditionally requiring human intelligence, such as pattern recognition, language understanding, prediction, and decision support. In clinical trials, AI is used to evaluate complex datasets, simulate trial scenarios, and recommend design and operational strategies.
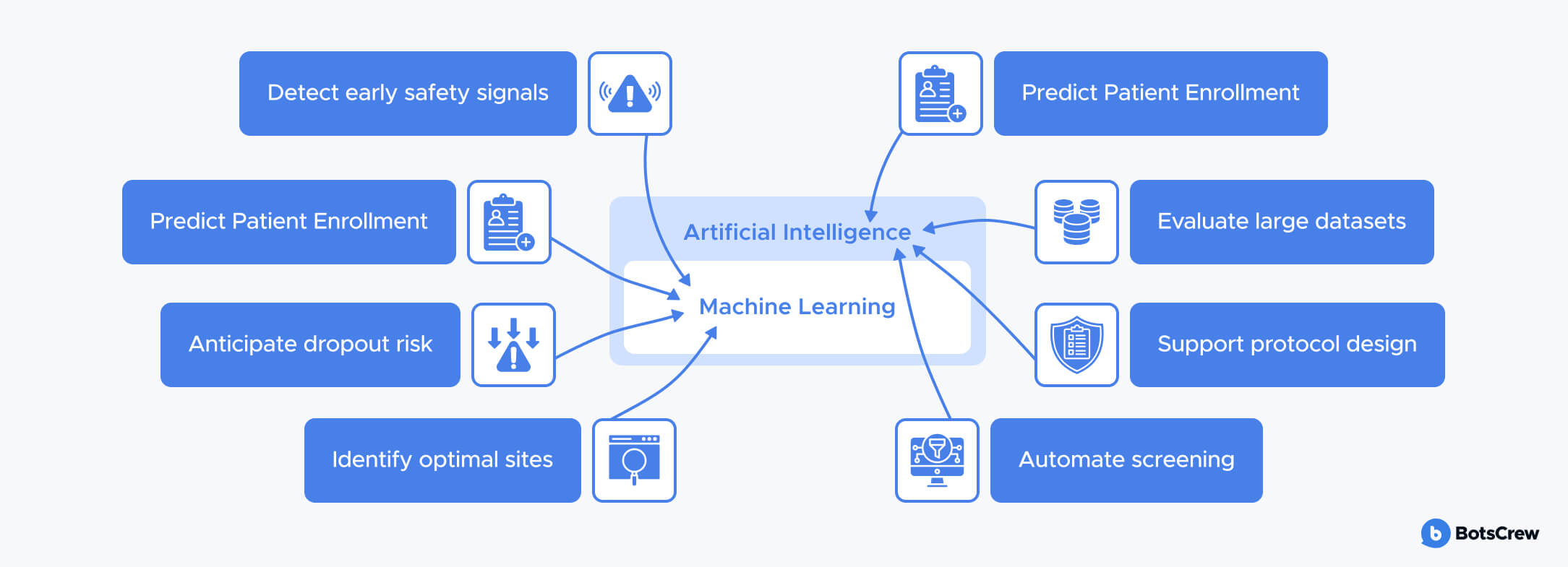
Machine Learning (ML) is a subset of AI focused on models that learn patterns from data and improve over time. In clinical research, ML is used to:
- Predict enrolment and dropout risk
- Identify optimal sites and geographies
- Detect early safety signals
- Model treatment response and variability
- Support synthetic and hybrid control arms
Together, AI and ML enable a shift from retrospective, assumption-driven trial design to prospective, data-driven simulation and optimization.
At BotsCrew, we view AI and ML not as replacements for clinical experts, but as strategic tools that amplify human judgment. When combined with strong domain knowledge and responsible implementation, they enable teams to design trials that are more feasible, efficient, and ultimately more likely to succeed.
See How AI & ML Apply to Your Current Pipeline
Not sure where predictive modeling, synthetic controls, or AI-driven recruitment fit into your trial portfolio?
Why Clinical Trials Need AI & ML Now
Clinical trials are at a breaking point. As the industry pushes toward more targeted therapies and data-rich trial environments, traditional design methods are struggling to keep up. This gap between what today’s science requires and what legacy processes can support is exactly why AI and ML are becoming indispensable.
Industry and academic analyses suggest that AI‑optimized trials can cut overall trial durations by roughly 20–30% and materially reduce operational costs by improving feasibility assessment, trimming unnecessary procedures, and forecasting recruitment risks before launch.
Rising R&D Costs and Longer Timelines
Precision medicine, biomarker-driven inclusion criteria, and multi-modal endpoints have increased protocol complexity. Each amendment, delay, or feasibility misjudgment compounds cost and risk. AI enables earlier detection of these issues - before the first patient is enrolled.
Patient recruitment and retention now account for an estimated 30% of overall drug development timelines, with delays costing sponsors between $600,000 and $8 million per day, depending on the program’s scale and market potential.
Explosion of Real-World Data (RWD)
Modern clinical research is surrounded by data: electronic health records, claims databases, imaging, biomarkers, genomic sequencing, wearables, and patient-reported outcomes. The challenge isn’t access - it’s making sense of it.
AI and ML can process massive, multimodal datasets at a scale that is impossible manually, uncovering patterns that help trial designers refine eligibility criteria, predict feasibility issues, and improve patient selection.
Shift Toward Precision Medicine
Today’s therapies are increasingly personalized, targeting specific biomarkers, genetic profiles, or patient subpopulations. This means trial designs must be more nuanced—and far more data-driven.
ML models help teams move beyond broad assumptions and instead simulate how different cohorts, endpoints, or protocol structures might perform. This results in trial designs that are more aligned with real clinical variability.
Growing Regulatory Acceptance of AI-Driven Models
Regulators like the FDA and EMA are becoming more supportive of AI’s role in trial design and execution. Recent guidance encourages the responsible use of AI/ML for patient identification, risk prediction, safety monitoring, and even synthetic control arms—provided models are transparent, validated, and well-documented.
This shift signals a major turning point: AI is no longer seen as experimental technology, but as a credible tool for increasing reliability and scientific rigor in clinical research.
Use Cases of AI & ML in Clinical Trials
AI and ML are now influencing decisions across the entire clinical trial lifecycle—from early protocol conception to safety oversight and regulatory submission. The greatest value comes when these capabilities are applied not as isolated tools, but as an integrated decision-support layer across design, feasibility, recruitment, execution, and analysis.
Below are the most impactful use cases, mapped to operational decisions and business outcomes.
Optimizing Protocols
Protocol design is one of the most critical determinants of clinical trial success, yet it is also one of the most common sources of delays and cost overruns. Poorly designed protocols drive slow recruitment, high screen-failure and dropout rates, protocol deviations, and costly amendments that can extend timelines by months or even years. Industry data show that a single substantial amendment can cost hundreds of thousands of dollars, and that up to nearly half of all amendments could be avoided with better upfront feasibility and design decisions.
AI and Machine Learning transform this front-loaded phase by replacing assumption-driven design with data-driven, predictive modeling. By learning from thousands of historical trials and large-scale real-world datasets, AI identifies which design elements truly add scientific value and which introduce unnecessary operational and patient burden.
Tufts CSDD data show that a single substantial protocol amendment costs, on average, about $141,000 in Phase II and around $535,000 in Phase III, not counting the much larger opportunity costs of delay.
Approximately 23-45% of amendments are considered avoidable through better initial protocol design (e.g., addressing feasibility, eligibility criteria, or recruitment challenges early).
AI and Machine Learning improve this front-loaded phase by using historical trials and real-world data to:
- Eliminate low-value complexity: Identify procedures and assessments that add burden without improving endpoints, reducing visit load and site workload.
- Predict real-world feasibility: Simulate recruitable population size, site capacity, enrollment speed, and dropout risk before study start.
- Optimize eligibility criteria: Balance scientific rigor with practical recruitability by testing how different criteria affect cohort size and diversity.
- Prevent costly amendments: Flag endpoints, visit schedules, and inclusion/exclusion rules that historically trigger protocol changes.
- Design for patient adherence: Use patient behavior and wearable data to optimize visit frequency, reduce fatigue, and improve retention.
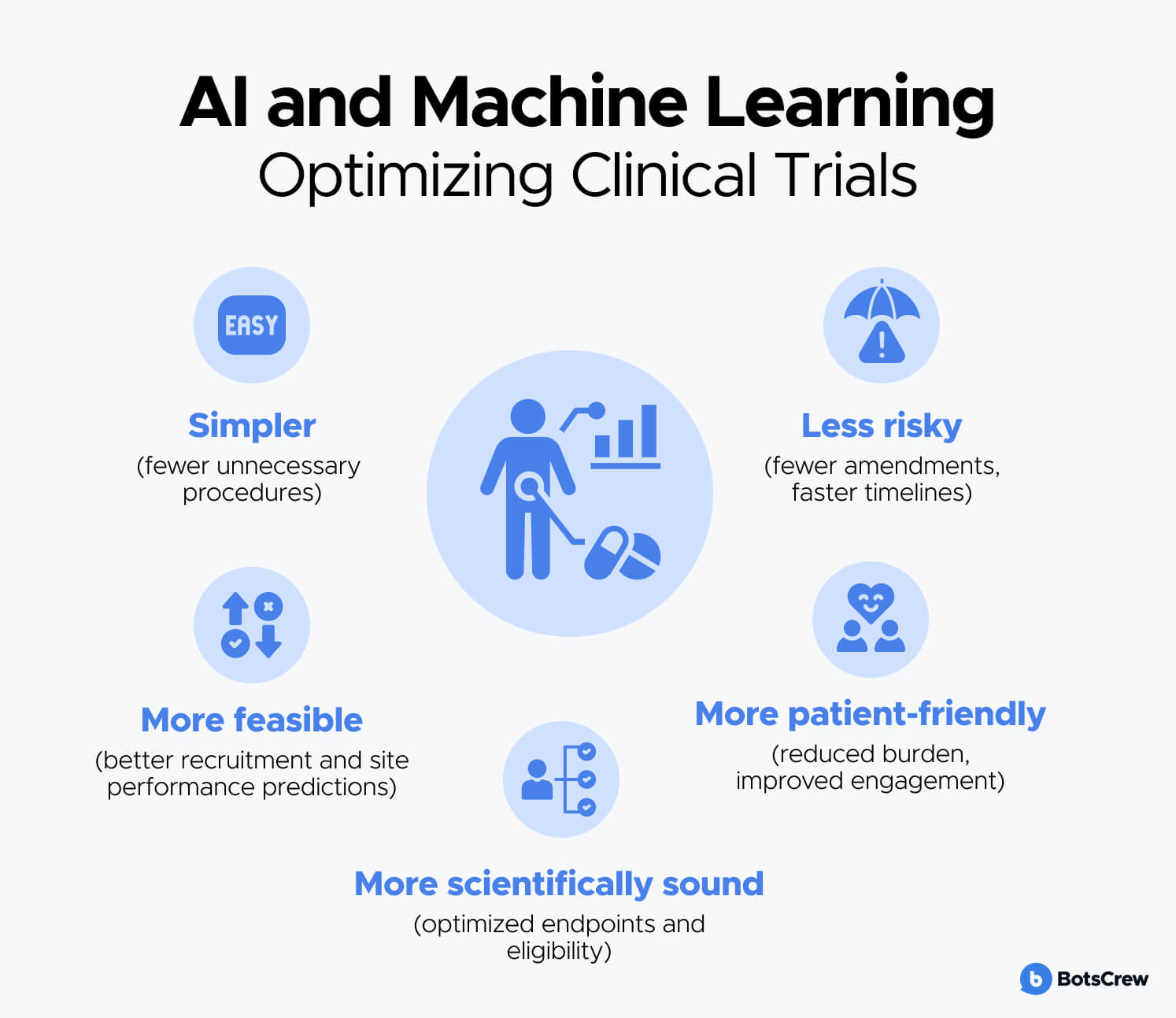
AI and ML optimize clinical trial protocols by making them:
- Simpler (fewer unnecessary procedures)
- More feasible (better recruitment and site performance predictions)
- More patient-friendly (reduced burden, improved engagement)
- More scientifically sound (optimized endpoints and eligibility)
- Less risky (fewer amendments, faster timelines)
Real World Example of Optimizing Protocols with AI
IQVIA's AI-Driven Protocol Benchmarking and Burden Reduction
IQVIA uses AI/ML on aggregated, de-identified real-world data from thousands of historical protocols to score and optimize new designs for complexity, patient burden, and site burden.
IQVIA has developed transformative technology that digitalizes protocols, allowing us to extract data and aggregate it in a de-identified fashion to gain granular insights from protocols based on specific diseases, trial phases, and therapeutic areas. They then apply AI/ML and scoring algorithms to quickly assess overall protocol complexity, patient burden, and site burden. Their continuously updated library of protocols allows for the creation of benchmarks against similar protocols, enabling sponsors to perform scenario planning and assess the impact of various design decisions. Armed with this knowledge, sponsors can make evidence-based decisions to reduce risk, streamline their design, and improve both patient and site experiences.
Accelerating Patient Recruitment
Patient recruitment is consistently one of the biggest reasons clinical trials run late—or never finish. Across the industry, ~80% of trials fail to recruit on their original timelines/targets, and a meaningful share fail to recruit enough patients to complete at all.
Even when sites are activated, enrollment performance is uneven: Tufts CSDD has reported that 11% of sites enroll zero patients and 37% under-enroll, while another Tufts CSDD analysis found 41% of sites were unable to achieve target enrollment.
AI and Machine Learning transform recruitment by replacing manual, site-by-site effort with proactive, data-driven patient finding, faster pre-screening, and predictive site/enrollment planning—so teams can identify the right patients earlier, activate the right sites, and stabilize enrollment curves.
AI and Machine Learning improve recruitment by enabling trial teams to:
- Find eligible patients faster: Match complex eligibility criteria against EHRs, claims, registries, genomics, and other real-world data at scale—shrinking what used to take weeks/months into days.
- Predict which sites will actually enroll: Use historical performance + local patient density + competing trials to forecast which sites/geographies will recruit fastest, reducing the risk of “activated but non-enrolling” sites.
- Automate pre-screening with NLP: Extract inclusion/exclusion signals from unstructured notes, labs, and reports—reducing coordinator workload and lowering screen-failure waste.
- Personalize outreach & improve engagement: Identify patients most likely to consent and stay enrolled; optimize channel/timing (SMS, email, portal) and tailor messaging for better response.
- Predict and prevent dropout: Detect early disengagement risk and trigger proactive support (reminders, visit optimization, follow-ups), improving retention and protecting timelines.
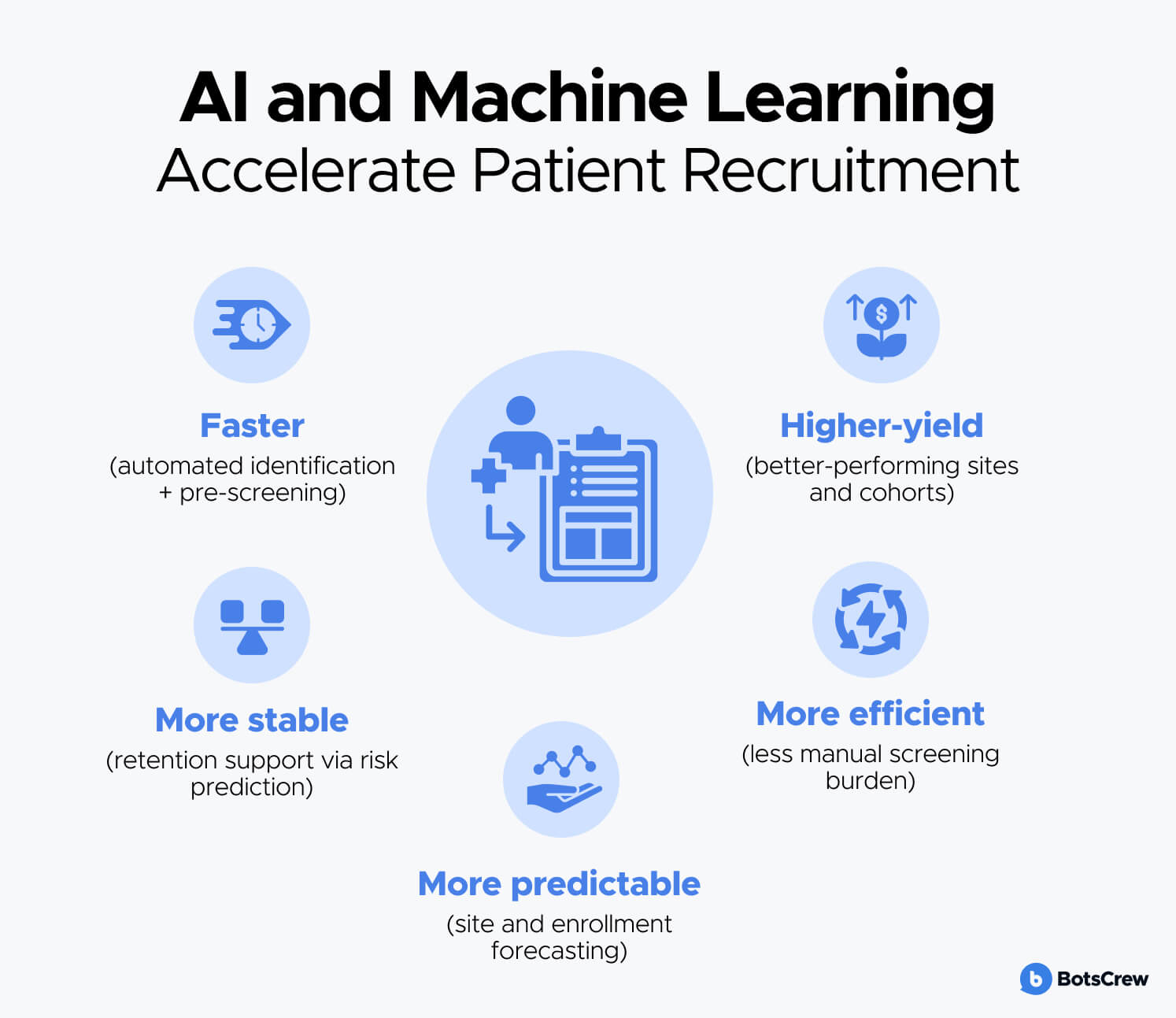
AI and ML accelerate patient recruitment by making it:
- Faster (automated identification + pre-screening)
- More predictable (site and enrollment forecasting)
- More efficient (less manual screening burden)
- More stable (retention support via risk prediction)
- Higher-yield (better-performing sites and cohorts)
Real World Example of AI-Driven Patient Recruitment
Clinical Trials Chatbot for Patients with CIU
Our client launched a clinical trial for chronic idiopathic urticaria (CIU) and faced the challenge of recruiting 2,000 highly qualified patients with strict eligibility criteria and a demanding visit schedule (19 visits over 68 weeks). To accelerate recruitment, an AI-powered conversational agent was deployed across Facebook and web channels to:
- Educate patients about trial requirements
- Pre-screen them through structured eligibility questions
- Automatically route qualified candidates to nearby study sites
Results:
- Over 20,000 targeted patients reached
- 395 qualified conversations initiated
- 41 highly eligible patients identified directly via the chatbot
- 2,000 total patients recruited for the study
This approach demonstrated how AI-driven pre-screening and digital engagement can significantly shorten the path from awareness to site referral while reducing manual screening burden.
Curify – AI-Powered Trial Matching & Pre-Screening Platform
Curify addresses one of the core inefficiencies in recruitment: the fact that a large share of screened patients fail to meet eligibility criteria, wasting time and resources. Using AI-driven matching, secure data infrastructure, and automated screener surveys, Curify connects patients to relevant trials and pre-qualifies them before site involvement.
Impact highlights:
- > 6,000 eligible patients referred to 12 clinical trials
- Over 200 patients successfully enrolled
- Access to a network of 1.5M+ research-interested participants
- Identification of 5,000+ eligible participants for a Moderna Phase III trial
- Support for large-scale vaccine studies (e.g., Moderna, Novavax) through rapid cohort identification and site coordination
By combining AI-based matching, large participant networks, and automated eligibility screening, Curify demonstrates how recruitment can shift from manual, site-limited processes to scalable, patient-centric digital pipelines.
Improving Diversity & Representation
Achieving representative trial populations is both a scientific necessity and an increasing regulatory expectation. Yet today, most clinical trials still fail to reflect real-world patient populations. In the U.S., for example, Black patients represent ~13% of the population but typically account for less than 5% of clinical trial participants, and similar gaps exist across age, gender, geography, and comorbidity profiles. Underrepresentation limits the generalizability of results, increases post-market safety risk, and slows regulatory confidence in new therapies.
These gaps are driven by structural barriers (site locations, access to care), protocol design (overly restrictive eligibility), and lack of trust and awareness in underserved communities. Traditional recruitment and site selection processes are not designed to systematically detect or correct these biases.
AI and Machine Learning help close these gaps by continuously analyzing population data, detecting bias in study design, and guiding inclusive site and outreach strategies—turning diversity from a late-stage reporting metric into a real-time operational objective.
AI and Machine Learning improve diversity and representation by enabling trial teams to:
- Identify representation gaps in real time: Compare enrolled populations against disease prevalence and target demographics (race, ethnicity, age, gender, geography, comorbidities) to detect underrepresented groups early and course-correct recruitment strategies.
- Detect bias in eligibility criteria: Use ML to surface hidden exclusion patterns in inclusion/exclusion rules (e.g., narrow lab thresholds, age cutoffs, comorbidity filters) and simulate alternative criteria that preserve scientific rigor while expanding access.
- Optimize inclusive site and geography selection: Map disease burden to population demographics and healthcare access, prioritizing community hospitals, regional clinics, and non-academic centers that serve minority and underserved populations—not only traditional research hubs.
- Tailor outreach and education to specific communities: Analyze language, cultural context, and information needs to personalize education and engagement, increasing awareness, trust, and trial participation among historically underrepresented groups.
AI and ML improve diversity and representation by making trials:
- More inclusive (systematic detection of demographic gaps)
- More equitable (reduced design and access bias)
- More generalizable (populations closer to real-world patients)
- More regulator-ready (stronger evidence across sub-groups)
- More trusted (community-aligned education and engagement)
Real World Example of AI-Driven Diversity Enablement
Clinical Trials & Treatment Education Chatbot for African-American Patients
The African-American population has the highest cancer mortality rates in the United States, yet has historically been underserved and underrepresented in oncology research. To improve access to information, trust, and participation in advanced treatments and clinical trials our client partnered with BotsCrew to deploy an AI-powered educational chatbot.
The chatbot was designed to:
- Educate Black patients and families about immunotherapy, precision oncology, and clinical trials
- Guide users toward tumor testing and trial participation
- Strengthen trust between patients, clinicians, and researchers through culturally sensitive language and tone
- Connect users with support resources and medical professionals
Results:
- 95% of users reported the chatbot was helpful
- 100% stated they clearly understood the information provided
- 85% Net Promoter Score, indicating strong trust and satisfaction
By combining AI-driven personalization, culturally adapted communication, and scalable digital access, the chatbot demonstrated how intelligent patient engagement can help address awareness gaps, build trust, and support more inclusive participation in oncology care and research.
Enhancing Safety Monitoring
Patient safety is the core objective of every clinical trial, yet traditional safety monitoring is still heavily manual, fragmented, and reactive. Adverse events are often reviewed retrospectively, across siloed systems, and at limited frequency, which can delay detection of emerging risks. Studies show that a large share of serious adverse events are reported days or weeks after onset, and manual case processing and coding can consume up to 60–70% of pharmacovigilance team effort.
As trials become more complex, decentralized, and data-rich (EHRs, wearables, ePROs, imaging, lab streams), human-only review is no longer sufficient to detect subtle, early safety signals at scale.
AI and Machine Learning enhance safety monitoring by shifting from periodic, manual review to continuous, real-time, and predictive surveillance—allowing risks to be detected earlier, across larger populations, and with greater sensitivity.
AI and Machine Learning improve safety monitoring by enabling trial teams to:
- Detect adverse events earlier: Continuously analyze EHRs, laboratory values, vital signs, and patient-reported outcomes to identify abnormal patterns and biomarker drifts before they escalate into serious safety signals.
- Identify real-time safety signals across sites and cohorts: Apply machine learning to detect emerging clusters of adverse events, distinguish true safety signals from background noise, and accelerate DSMB and pharmacovigilance decision cycles.
- Extract safety insights from unstructured data: Use Natural Language Processing (NLP) to scan clinical notes, call transcripts, and patient diaries to identify symptoms, side effects, and severity in near real time—dramatically reducing manual case review workload.
- Automate medical coding and regulatory reporting: Auto-code adverse events to MedDRA, generate SAE and SUSAR reports, and support expedited submissions—improving compliance and reducing reporting delays.
AI and ML enhance safety monitoring by making it:
- Earlier (continuous signal detection instead of periodic review)
- More sensitive (ability to detect subtle trends and rare events)
- More scalable (analysis across thousands of patients and data streams)
- More efficient (automation of review, coding, and reporting)
- More compliant (faster, more consistent regulatory submissions)
Real World Example of AI-Driven Safety & Clinical Decision Support
AI Assistant for Real-Time Clinical Information Access
Together with our client, we created an AI-powered assistant designed to support healthcare professionals with instant, secure access to patient histories, medications, care plans, and clinical guidelines across web and mobile platforms. While deployed in real-world care settings, its architecture and capabilities closely mirror the needs of safety monitoring and decision support in clinical trials.
Key safety-relevant capabilities include:
- Real-time access to patient-specific data: Clinicians can instantly query current medications, conditions, and care plans, reducing the risk of medication errors and missed contraindications.
- NLP over unstructured medical documentation: The system can retrieve and interpret information from clinical notes, policies, and care records, enabling faster identification of symptoms, treatments, and potential safety issues.
- Secure, role-based patient data separation: Dedicated patient-specific chats ensure that sensitive medical information is accessed only by authorized professionals, supporting regulatory compliance and data integrity.
- Decision support at the point of care: By providing immediate, evidence-based answers and clinical context, our solution helps reduce delays, miscommunication, and oversight that can contribute to adverse events.
Impact highlights:
- 24/7 access to critical patient information
- Significant reduction in time spent searching fragmented records
- Improved accuracy and confidence in clinical decision-making
- Strong user trust and adoption across care teams
This case demonstrates how AI-driven real-time data access, NLP over clinical documentation, and automated knowledge retrieval can form the foundation of continuous safety surveillance—capabilities directly transferable to clinical trial safety monitoring, pharmacovigilance workflows, and DSMB support.
Reducing Control Arm Burden
Control arms are essential for scientific validity, but they also represent one of the greatest ethical and operational challenges in clinical trials. Large numbers of patients may be randomized to placebo or standard-of-care, even when promising therapies exist. Control groups also drive sample size, cost, and trial duration—often accounting for 40–60% of total enrollment in late-phase studies.
As precision medicine and rare-disease research expand, recruiting large control cohorts becomes increasingly difficult and ethically sensitive. Regulators are also encouraging innovative designs that reduce unnecessary patient exposure while maintaining statistical rigor, particularly in oncology, rare diseases, and pediatric trials.
AI and Machine Learning are transforming control arm design by enabling data-driven alternatives to traditional placebo groups, such as synthetic controls, external real-world comparators, and adaptive randomization—allowing trials to reach conclusions faster with fewer patients assigned to less effective treatments.
AI and Machine Learning reduce control arm burden by enabling trial teams to:
Create synthetic / virtual control arms
Leverage historical clinical trials, real-world evidence (RWE), and EHR data to model expected outcomes and build “digital twins” of control patients, reducing the number of participants randomized to placebo while preserving statistical power.
Integrate external real-world control cohorts
Align real-world patient populations to trial inclusion/exclusion criteria and adjust for confounders using causal inference, propensity scoring, and matching—allowing external controls to supplement or partially replace traditional control groups, especially in rare and oncology indications.
Enable adaptive randomization
Continuously analyze accumulating data to shift randomization toward better-performing treatment arms and stop underperforming control arms earlier, reducing exposure to less effective therapies and improving ethical balance.
Accelerate interim analyses and decision-making
Predict treatment effect trajectories and safety trends in real time, enabling earlier go/no-go decisions, faster futility stopping, and shorter overall trial duration.
AI and ML reduce control arm burden by making trials:
- More ethical (fewer patients on placebo or inferior therapy)
- Faster (earlier efficacy and futility signals)
- Smaller (reduced sample size requirements)
- More efficient (lower cost per endpoint achieved)
- More regulator-aligned (support for innovative and hybrid control designs)
Real World Examples of AI-Enabled Control Arm Innovation
Roche & Flatiron Health – External Real-World Control Arms in Oncology
Roche has used real-world data from Flatiron Health to construct external control cohorts for oncology studies, enabling comparison against historical and real-world standard-of-care populations. These AI- and ML-adjusted control arms have supported regulatory submissions and helped reduce the need for large placebo groups, particularly in rare cancer subtypes.
FDA-Approved Use of Synthetic Controls in Rare Disease Trials
In multiple rare-disease programs (e.g., Duchenne muscular dystrophy, ALS, and pediatric oncology), regulators have accepted synthetic control arms built from natural history data and EHRs, with ML-based matching and bias adjustment. These approaches reduced required control enrollment by 30–60% while maintaining statistical validity and accelerating approval timelines.
Adaptive Platform Trials (e.g., I-SPY 2, RECOVERY, REMAP-CAP)
AI-supported adaptive designs continuously learn from incoming data, dynamically reassigning patients to more effective arms and dropping underperforming controls early. In I-SPY 2 (breast cancer), this approach shortened time to identify successful therapies and reduced patient exposure to ineffective treatments by reallocating enrollment in near real time.
Challenges & Limitations
While AI and Machine Learning offer significant promise across protocol design, recruitment, safety monitoring, and trial execution, their adoption in clinical research also introduces important technical, operational, and regulatory challenges. Understanding these limitations is critical to ensuring that AI-driven systems are used responsibly, transparently, and in a way that supports—not replaces—scientific and clinical judgment.
1. Data Quality, Bias, and Representativeness
AI models are only as reliable as the data they are trained on. Clinical trial and real-world datasets often suffer from missing values, inconsistent coding, site-level variability, and historical biases. If underrepresented populations are poorly captured in training data, models may perpetuate or amplify existing disparities in eligibility screening, risk prediction, or safety signal detection. This can lead to skewed recommendations, reduced generalizability, and regulatory concern.
2. Limited Interoperability and Data Silos
Clinical trial data are fragmented across EHR systems, ePRO platforms, imaging repositories, lab systems, and sponsor databases. Lack of standardized formats and semantic alignment makes it difficult to integrate these sources into unified AI pipelines. Without robust interoperability, AI systems may operate on partial or delayed data, reducing their predictive accuracy and real-time value.
3. Model Transparency and Explainability
Regulators, clinicians, and ethics committees require clear understanding of how decisions are made—especially when AI influences patient eligibility, safety assessment, or dose escalation. Many advanced ML models (e.g., deep neural networks) function as “black boxes,” making it difficult to explain why a particular prediction or recommendation was generated. Limited interpretability can slow regulatory acceptance and reduce clinician trust.
4. Regulatory and Validation Complexity
AI systems used in clinical trials must meet strict validation, documentation, and auditability requirements. Demonstrating that a model is reliable, unbiased, stable across populations, and fit for its intended use is a non-trivial process. In adaptive or continuously learning systems, maintaining a validated state while models evolve over time poses additional regulatory challenges.
5. Privacy, Security, and Data Governance
AI-driven trials often rely on large volumes of sensitive health data, including EHRs, genomics, and wearable streams. Ensuring compliance with regulations such as HIPAA, GDPR, and emerging AI governance frameworks requires strong encryption, access controls, data minimization, and secure model training environments. Breaches or misuse can erode patient trust and create legal risk.
6. Operational Integration and Change Management
Embedding AI into clinical operations requires workflow redesign, staff training, and cross-functional alignment between clinical, data, IT, and regulatory teams. Poor integration can lead to duplicated work, alert fatigue, or overreliance on automated outputs without proper human oversight.
7. Need for Human Oversight and Clinical Judgment
AI excels at pattern recognition and prediction, but it does not understand clinical context, patient values, or evolving medical standards in the same way humans do. Critical decisions—such as protocol amendments, safety actions, or go/no-go calls—must remain under expert supervision, with AI serving as decision support rather than an autonomous authority.
In summary, while AI and ML can significantly enhance the efficiency, speed, and quality of clinical trials, their successful deployment depends on high-quality data, transparent models, regulatory-aligned validation, strong governance, and close collaboration between technology teams and clinical experts. Addressing these challenges is essential to unlocking the full, responsible potential of AI in clinical research.
Ready to Design Smarter, Faster, More Patient-Centric Trials with AI?
At BotsCrew, we help life sciences teams move from experimentation to production-grade, regulator-ready AI - across protocol design, recruitment, safety, and trial operations.
Whether you are:
- Planning your first AI-enabled trial,
- Scaling across a portfolio,
- Or modernizing legacy clinical systems
we help you move from strategy to deployment.
Book a Free AI Strategy Session for Clinical Trials








